Chinese language drug 3D printing agency Triastek has introduced that its T22 3D printed gastric retention product has develop into the primary drug of its type to obtain Investigational New Drug (IND) clearance from the US Meals and Drug Administration (FDA).
T22 has been filed beneath part 505(B)(2) of the FDA’s Federal Meals, Drug, and Beauty Act. The 3D printed drug is designed to deal with pulmonary arterial hypertension (PAH) and power thromboembolic pulmonary hypertension (CTEPH).
In keeping with Triastek, this new product considerably reduces dosage frequency from thrice a day to only as soon as a day. That is stated to simplify the dosing routine and enhance remedy adherence.
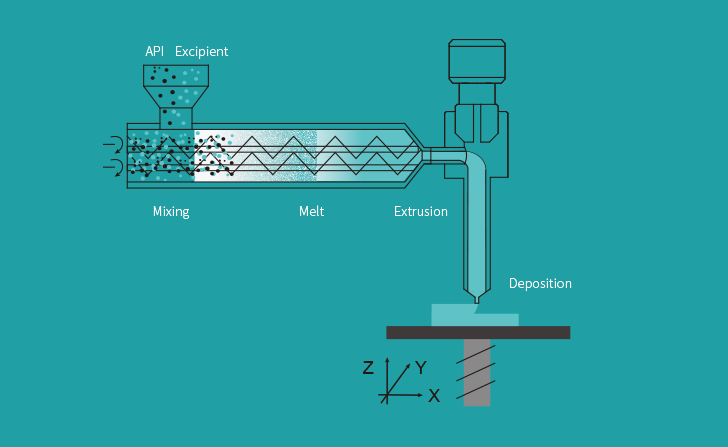
T22 is 3D printed utilizing Triastek’s Soften extrusion Deposition plus Micro-Injection Molding (MED&MIM) course of and incorporates its 3D Microstructure for Gastric Retention (3DµS-GR) for optimized drug supply.
That is the fourth Triastek 3D printed drug to obtain FDA clearance to the medical improvement stage, including to the corporate’s T-series portfolio of T19, T20 and T21 merchandise. As such, the corporate claims to rank first within the world 3D printed drug subject by way of improvement product depend.
Triastek is now making ready to provoke medical trials with T22 to fast-track the product’s improvement.
“Based mostly on our proprietary 3D Microstructure for Gastric Retention supply expertise platform, the 2 merchandise we developed, T20G and T22, have acquired IND clearance to proceed from regulatory companies in China and the US this 12 months, marking the profitable first step for Triastek’s this revolutionary supply expertise platform continuing via regulatory overview course of,” acknowledged Triastek founder and CEO Dr. Senping Cheng.


MED 3D printed prescription drugs
Since launching in 2015, Triastek has constructed a enterprise solely centered on the manufacturing of 3D printed stable dosage kind medication. Triastek boasts 213 patent functions associated to its 3D printed prescription drugs throughout 10 international locations, 68 of which have been granted.
The corporate’s proprietary MED extrusion-based 3D printing expertise gives an end-to-end methodology for manufacturing a variety of novel dosage kind designs. The system repeatedly converts powder feedstocks into softened states. This molten materials is then deposited layer-by-layer to supply bodily medication with optimized geometric constructions.
Such constructions could be in any other case unimaginable to supply. As such, the 3D printing course of permits for drug launch management at a degree that may’t be replicated with typical pill manufacturing strategies.

T22: Triastek’s latest 3D printed drug
Again in 2021, Triastek signed a co-development settlement with Sperogenix Therapeutics to develop and commercialize the T22 in East Asia. This partnership sought to show the medical utility worth of 3DµS-GR.
In keeping with Dr. Cheng, the next progress of the T22 has led to firms from a number of international locations and areas to precise curiosity in potential collaborations for product improvement using the 3DµS-GR drug supply platform.
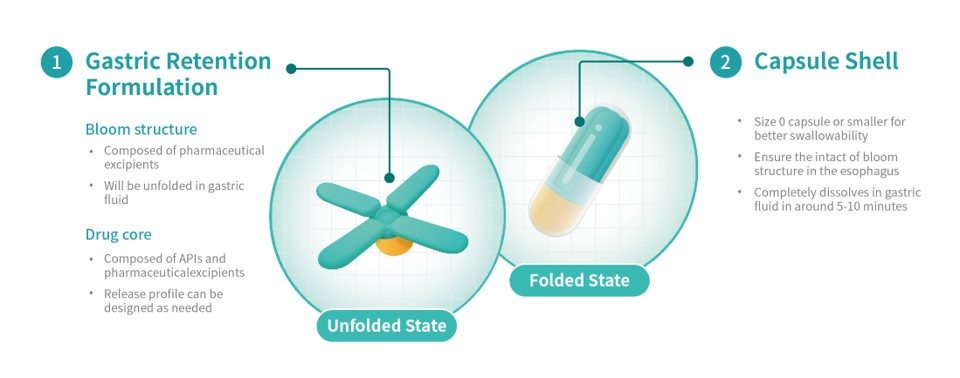
Triasktek has now submitted a Patent Cooperation Treaty (PCT) utility for its 3DµS-GR supply expertise, and the distinctive Bloom Construction design of the T22.
Following oral administration, the T22 gastric retention prototype expands to a measurement bigger than the pylorus, the opening from the abdomen to the small gut. This extends gastric retention time. Throughout the gastric retention interval, the T22 releases APIs based on predetermined programmed drug launch habits.
Along with simplifying the dosage routine, decreasing remedy burden, and bettering long-term remedy adherence, this course of may enhance drug absorption and oral bioavailability.
Triastek has now accomplished improvement of the T22 gastric retention formulation. This formulation is claimed to have achieved constructive outcomes by way of in vitro growth time, mechanical energy and dissolution habits.
The corporate has additionally accomplished Pharmacokinetic (PK) research of the T22 prototype on beagle canine. The PK examine assessed how the physique interacts with the drug all through publicity. Right here the corporate demonstrated that one every day dose of the T22 prototype gave comparable PK parameters as thrice a day dosing of the unique product.

Developments in pharmaceutical 3D printing
In current surveys on the way forward for 3D printing and 3D printing traits, 3D printing consultants highlighted developments in medical functions as being a key space of present trade development that can proceed within the coming years.
Certainly, Triastek is just not the one firm to make notable developments within the 3D printing pharmaceutical drug supply area.
Final 12 months, a analysis group from the Max Planck Institute for Informatics in Saarbrücken, Germany, and the College of California at Davis developed novel 3D printed drugs that may launch pharmaceutical medication at predetermined speeds. In a analysis paper, the group demonstrated how the drugs might be 3D printed with particular shapes which decide the velocity at which they dissolve within the human physique.
Provided that geometric shapes are simpler to regulate than various timed drug-delivery strategies akin to intravenous infusion, the researchers declare that this new methodology possesses vital potential past pharmaceutics. For example, the group claims that the manufacturing of catalytic our bodies and coarse granular fertilizers could also be further functions of their findings.
Equally, in 2016 Aprecia Prescribed drugs introduced that their Spritam remedy had develop into the primary 3D printed pharmaceutical to obtain FDA approval. Spritam is designed to deal with a spread of seizures and is 3D printed as an immediately dissoluble pill. This makes the remedy extra accessible to sufferers who would in any other case wrestle to swallow the tablet.
Subscribe to the 3D Printing Business publication to maintain updated with the most recent 3D printing information. It’s also possible to comply with us on Twitter, like our Fb web page, and subscribe to the 3D Printing Business Youtube channel to entry extra unique content material.
Are you curious about working within the additive manufacturing trade? Go to 3D Printing Jobs to view a number of accessible roles and kickstart your profession.
Featured picture reveals Triastek’s T19 drug for rheumatoid arthritis. Photograph by way of Triastek.


